Cooperativity
at the
Molecular Main Group – Transition metal
Interface
Cooperativity is a powerful thing. Sometimes we have to work together to get a job done efficiently. The same can also be true in chemistry: nature has evolved to use multiple subtle interactions to activate and functionalise even the most inert molecules!
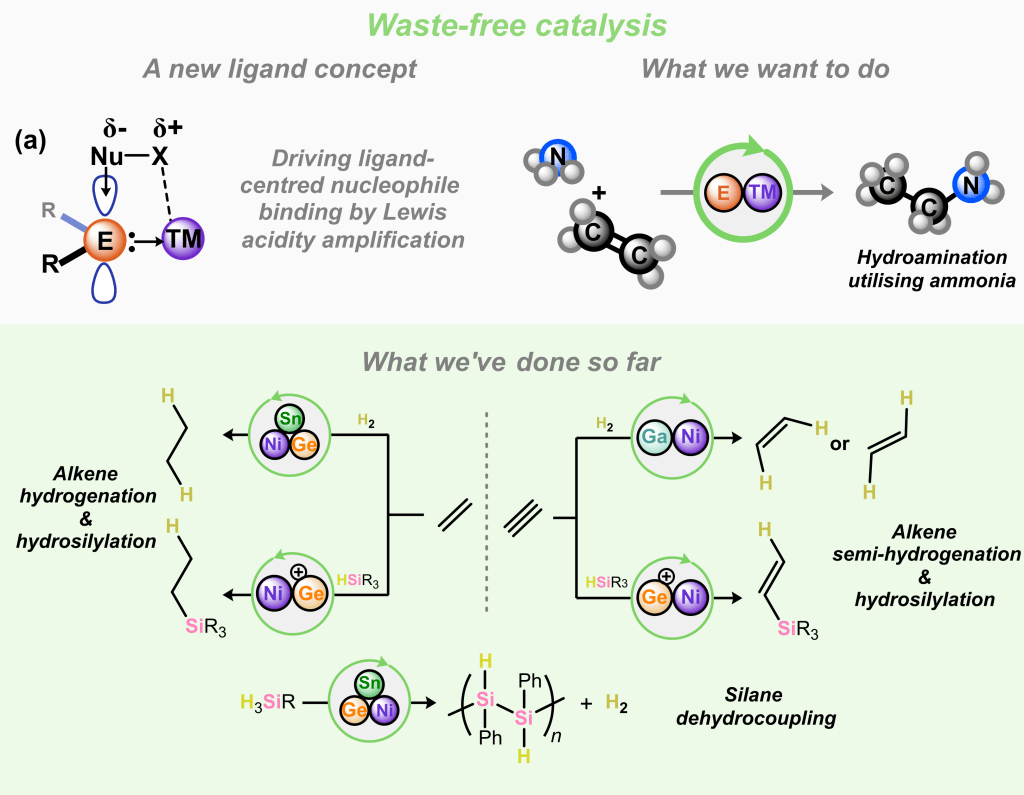
In our work the develop what we call the ‘Single-Centre Ambiphile’ ligands. These have a single binding centre which is simultaneously Lewis acidic and Lewis basic. This latter functionality binds a metal centre through a classic L-type donor interaction. The Lewis acidic functionality, through some nifty ligand design, remains highly electrophilic, allowing for the binding of nucleophiles! This opens up new pathways in cooperative bond activation, using this ligand-centred functionality in conjunction with the transition metal.
In addition to this, we mostly study abundant first row transition metals, looking towards more sustainable catalytic systems. These can be hard to control, in terms of reactivity, as they typically have numerous accessible energetic states. Our ligands help to tame this high reactivity, whilst also getting involved in bond activation themselves.
So far, we’ve demonstrated reversible ligand centred ammonia binding and activation, reversible and cooperative H2 activation, as well as waste-free catalytic alkene functionalisation, even whith ‘switchable’ catalysts. This is just the tip of the iceberg, and we look forward to sharing all the exciting results coming our way soon…